Ruben Sauleda

The following article first appeared in the American Orchid Society BULLETIN in April of 1976. It has been edited to reflect current nomenclatural concepts
The early work of Knudson (1922, 1924 and 1925), demonstrating that orchid embryos could be grown in vitro without the intricacies of a host-fungal relationship, has played an essential role in advancing orchidology. The hybridizing potential found in orchids, both at the inter-specific and inter-generic level, has been utilized by amateur and commercial orchid growers to produce thousands of artificial hybrids. The desire to observe the resulting hybrids quickly leads to research and advances in in vitro culture techniques. The availability of large numbers of orchid seeds, with little or no food reserve and relatively uniform cultural and genetic characteristics, has prompted researchers to use orchid seeds for in vitro nutritional and developmental studies. These studies have led to many innovations in in vitro culture techniques.
Refinements in aseptic technique and equipment (Sauleda, 1970) have lowered the rate of contamination during seed sowing and replating. The development of specialized culture media (Withner, 1959), both defined and undefined, for germination of orchid seeds and replating has increased the number of seeds germinating and accelerated seedling growth rates. These advances and developments have contributed to a substantial reduction in flowering time. Phalaenopsis Blume, Oncidium Swartz and Dendrobium Swartz, usually requiring four to five years to flower from seed sowing, can now be regularly flowered in less than two years.
Another advancement increasing the germination of orchid seeds and reducing flowering time was the development of a green pod culture process (Tsuchiya-Itaru, 1954). In most laboratories this process has replaced, whenever possible, the dry seed culture process.
In the dry seed process, the seed capsule is removed from the plant at the first sign of dehiscence. The seeds are separated from the seed capsule, treated with a sterilizing agent and sown using aseptic procedure. A large number of seeds may be lost by contamination as a result of incomplete seed sterilization. Overexposure to the sterilizing agent may also cause excessive seed loss due to burning. The time required by the sterilizing agent to decontaminate the seeds without burning the proto-embryo is sometimes difficult to estimate. The effect of the sterilizing agent may vary depending on the genus and the age of the seeds being sown.
In the green pod culture process, the seed capsule is removed from the plant after fertilization, but prior to dehiscence. The surface of the seed capsule is decon-taminated with a sterilizing agent, the seed capsule is opened and the seeds removed and sown using aseptic procedure. During this process the seeds are not contaminated by exposure to air, eliminating the need for contact with a sterilizing agent and the possibility of burning the seed. The result is an increase in the number of seeds germinated.
The difference in harvesting time between the dry seed process and the green pod culture process may be as much as six to eight months in some genera. This reduction in harvesting time decreases the time required for flowering.
Harvesting time for optimal germination is a factor which must be investigated and controlled. The seed capsule should be removed from the plant after fertilization, but prior to dehiscence. This paper will show the results of investigations establishing harvesting time for optimal germination of orchid seeds using the green pod culture process.
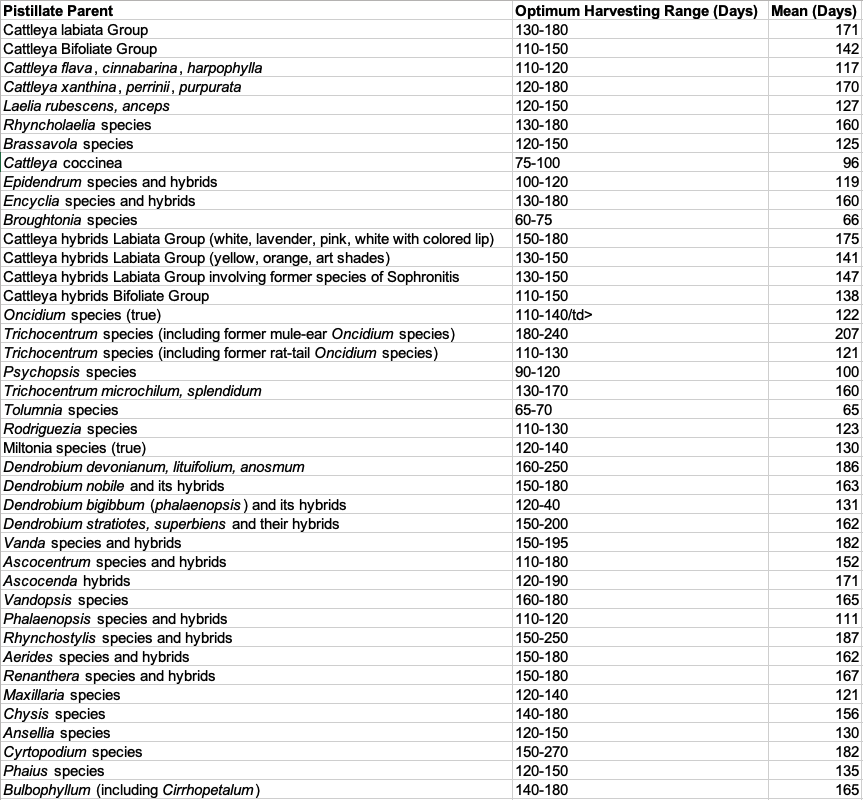
Hybridizing records accumulated during a fifteen-year period were used to determine the approximate optimal harvesting time for 28 genera. The minimum harvesting time was determined for each species group by using the least number of days at which good germination occurred. The maximum was determined by using the maximum number of days at which good germination occurred. In each case the mean was calculated from a minimum of four seed capsules.
Harvesting Times for Selected Species and Hybrids
RESULTS AND DISCUSSION
Results of crosses made between plants with differing harvesting times indicate that the pistillate parent is mainly responsible for determining the harvesting time.
The harvesting time for a bifoliate Cattleya was experimentally determined to be 120-150 days. When reciprocal crosses were made, the harvesting time for the crosses coincided with the harvesting time of the pistillate parent. When the bifoliate Cattleya was the pistillate parent, the harvesting time was 120-150 days, and, when the unifoliate Cattleya was the pistillate parent, the harvesting time was 150-180 days. Reciprocal crosses between Vanda and Ascocenda hybrids and between Oncidium Alliance species with different harvesting times also indicated that the pistillate parent determined the harvesting time.
Optimal harvesting time was found to be specific not only to genera and hybrids but to species and in some cases to individual plants.
The time intervals listed are meant as general guides for hybridizers attempting similar crosses for the first time. Harvesting times vary as a result of complex interrelations between genetic and environmental factors. Individual breeders must make adjustments to compensate for prevailing local conditions. - 72500 S. W. 46th Street, Miami, Florida 33175.
REFERENCES
Knudson, L. 1922. Non-symbiotic germination of orchid seeds. Bot. Gaz. 73: 1-25. 1924. Further observations on non-symbiotic germination of orchid seeds. Bol.Gat. 77: 212-219. 1925. Physiological study of the symbiotic germination of orchid seeds. Bot. Gat. 79: 345-379.
Sauleda, R. P. 1970. An aseptic transfer case based on a liquid seal. Amer. Orchid Soc. Bull. 39:515-516.
Tsuchiya, Itaru. 1954. Germination of orchid seeds from premature pods. Na Pua Okika o Hawaii Met. 4:11-16.
Withner, C. L. (Ed.). 1959. The Orchids. The Ronald Press Co., New York.









